The Most Common Equine Parasites And The Damage They Cause.
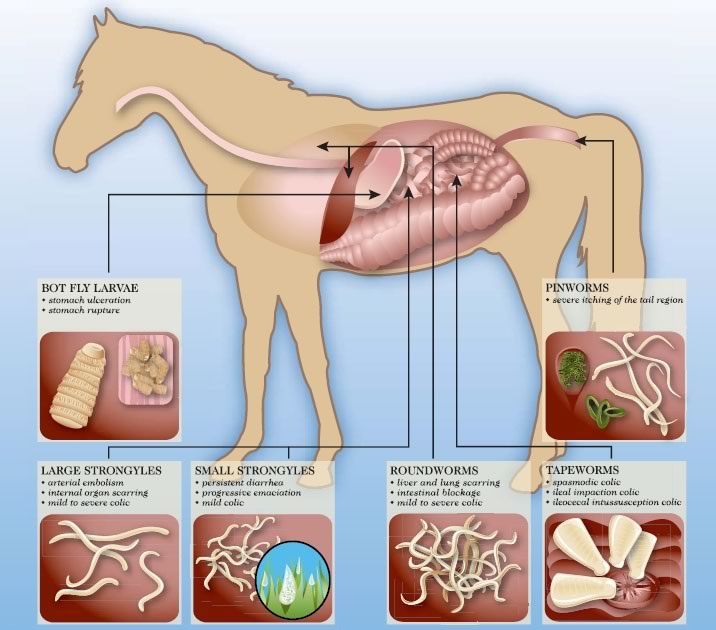
Use the diagram or the parasite menu to learn more about each parasite.
Bimectin Horse Wormer (Ivermectin) is an oral paste wormer for horses containing ivermectin as it's active ingredient. Animec treats a range of internal parasites, including small and large redworms, lungworms, pinworms and bots in horses.
Active Ingredient: Ivermectin
Target Species: Horse
Treats and Controls: Large redworms, Small redworms, Pinworms, Lungworms, Ascarids, Stomach worms, Intestinal threadworms, Cutaneous worms, Bots
Administration Method: Oral paste
Withdrawal Time: Withdrawal period for meat and offal is 34 days. Do not use in mares producing milk for human consumption.
Dosage for Horses: Each syringe is sufficient to dose a horse up to 600kg.
Always read the label and all enclosed information for Bimectin Horse wormer before administering to animals!
Use the diagram or the parasite menu to learn more about each parasite.

Click here to Download Data Sheet
Health Products Regulatory Authority
Summary of Product Characteristics
1 NAME OF THE VETERINARY MEDICINAL PRODUCT
Bimectin Horse Oral Paste 18.7 mg/g
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active substance:
Ivermectin 18.7 mg/g
For a full list of excipients see section 6.1.
3 PHARMACEUTICAL FORM
Oral paste.
A yellow, gel-like paste of uniform consistency.
4 CLINICAL PARTICULARS
4.1 Target Species
Horses.
4.2 Indications for use, specifying the target species
The product is indicated for the treatment of nematode or arthropod infestations in horses due to:
Large strongyles
Strongylus vulgaris (adults and 4th larval [arterial] stages)
S. edentatus (adults and 4th larval [tissue] stages)
S. equinus (adults)
Triodontophorus spp. (adults)
Triodontophorus brevicauda
Triodontophorus serratus
Small Strongyles Adults and immatures (fourth stage larvae) small strongyles or cyathostomes unless otherwise stated. Ivermectin is not effective against the encysted larval stages of the small strongyles.
Coronocyclus spp.
Coronocyclus coronatus
Coronocyclus labiatus
Coronocyclus labratus
Cyathostomum spp.
Cyathostomum catinatum
Cyathostomum pateratum
Cylicocyclus spp. Cylicocyclus ashworthi Cylicocyclus elongatus Cylicocyclus insigne Cylicocyclus leptostomum Cylicocyclus nassatus
Cylicostephanus spp.
Cylicostephanus calicatus
Cylicostephanus goldi
Cylicostephanus longibursatus
Cylicostephanus minutus
Cylicodontophorus spp.
Cylicodontophorus bicornatus
Parapoteriostomum spp.
Parapoteriostomum mettami
Petrovinema spp.
Petrovinema poculatum
Poteriostomum spp.
Lungworms (adult and inhibited fourth stage larvae)
Dictyocaulus arnfieldi
Pinworms (adult and inhibited fourth stage larvae)
Oxyuris equi
Ascarids (adults and third & fourth stage larvae)
Parascaris equorum
Hairworms (adults)
Trichostrongylus axei
Large-mouth stomach worms (adults)
Habronema muscae
Neck threadworms (microfilariae)
Onchocerca spp.
Intestinal threadworms (adults)
Strongyloides westeri
Stomach bots (oral and gastric stages)
Gasterophilus spp.
4.3 Contraindications
None.
4.4 Special warnings for each target species
Care should be taken to avoid the following practices because they increase the risk of development of resistance and could ultimately result in ineffective therapy:
Too frequent and repeated use of anthelmintics from the same class, over an extended period of time.
Underdosing, which may be due to underestimation of body weight or misadministration of the product.
Suspected clinical cases of resistance to anthelmintics should be further investigated using appropriate tests (e.g. Faecal Egg Count Reduction Test).
Where the results of the tests(s) strongly suggest resistance to a particular anthelmintic, an anthelmintic belonging to another pharmacological class and having a different mode of action should be used.
Resistance to ivermectin has been reported in Parascaris equorum in horses in a number of countries, including the EU. Therefore, the use of this product should be based on local farm epidemiological information about susceptibility of nematodes and recommendations on how to limit further selection for resistance to anthelmintics.
4.5 Special precautions for use
(i) Precautions for use in animals
Special warning for non-target species:
The product has been formulated for use in horses only.
Cats, Dogs, especially Collies, Old English Sheepdogs and related breed or crosses, and also turtles and tortoises may be adversely affected by the concentration of ivermectin in this product if they are allowed to ingest spilled paste or have access to used syringes.
Parasite resistance to any particular class of anthelmintic may develop following frequent, repeated use of an anthelmintic of that class.
(ii) Special precautions to be taken by the person administering the veterinary medicinal product to animals
Do not eat, drink or smoke while handling the product.
Avoid contact with skin and eyes.
If accidental skin contact occurs, wash the affected area immediately with soap and water.
If accidental eye exposure occurs, flush the eyes immediately with water and, if necessary, get medical attention. Wash hands after use.
4.6 Adverse reactions (frequency and seriousness)
Some horses carrying heavy infection of Onchocerca microfilariae have experienced oedema and pruritus following dosing, assumed to be the result of death of large numbers of microfilariae.
These signs resolve within a few days but symptomatic treatment may be advisable.
4.7 Use during pregnancy, lactation or lay
Studies performed in laboratory animals showed no teratogenic or embryotoxic effect of ivermectin at the recommended doses during therapy.
The safety of the veterinary medicinal product has not been established during pregnancy and lactation.
Use only according to risk/benefit analysis by the responsible veterinary surgeon.
Please refer also to 4.11.
4.8 Interaction with other medicinal products and other forms of interactions
The effects of GABA agonists are increased by ivermectin.
4.9 Amounts to be administered and administration route
Administer orally as a single dose rate to horses at the recommended dose level of 0.2 mg ivermectin per kilogram of bodyweight.
The smaller syringe delivers 120 mg ivermectin, sufficient to treat 600 kg of bodyweight.
The bigger syringe delivers 160 mg ivermectin, sufficient to treat 800 kg of bodyweight.
To ensure administration of the correct dose,body weight should be determined as accurately as possible.
If animals are to be treated collectively rather than individually, they should be grouped according to their bodyweight and dosed accordingly, in order to avoid under- or over-dosing.
This is a single dose product.
Discard after use.
Dosing Instructions:
Each weight marking on the syringe plunger will deliver sufficient paste to treat 100 kg bodyweight.
Unlock the knurled ring by making ¼ turn and slide the knurled ring up the plunger shaft so that the side nearest the barrel is at the prescribed weight marking.
Turn the knurled ring ¼ turn to lock in place.
Make sure the horse’s mouth contains no feed.
Remove the plastic cap from the tip of the nozzle.
Insert the syringe into the horse’s mouth at the interdental space.
Advance the plunger as far as it will go, depositing the medication on the base of the tongue.
Immediately raise the horse’s head for a few seconds after dosing.
The treatment schedule should be based on the local epidemiological situation.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
Mild transitory signs (slowed pupillary light response and depression) have been seen at a dose of 1.8mg/kg (9 times the recommended dose level).
Other signs seen at higher doses includes mydriasis, ataxia, tremors, stupor, coma and death.
The less severe signs have been transitory.
No antidote has been identified; however, symptomatic therapy may be beneficial.
4.11 Withdrawal period(s)
Meat and offal: 34 days.
Do not use in mares producing milk for human consumption.
5 PHARMACOLOGICAL or IMMUNOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group:
Endectocide
ATC vet code:
QP54AA01
Ivermectin is a member of the macrocyclic lactone class of endectocides.
Compounds of the class bind selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerve and muscle cells.
This leads to an increase in the permeability of the cell membrane to chloride ions and hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of theparasite.
Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The margin of safety for compounds of this class is attributable to the fact that mammals do not have glutamate-gated chloride channels, the macrocyclic lactones have a low affinity for other mammalian ligand-gated chloride and they do not readily cross the blood-brain barrier.
5.2 Pharmacokinetic particulars
Following administration of the product, ivermectin is rapidly absorbed to reach peak plasma concentration in several hours.
This peak falls off gradually over several days. Ivermectin is eliminated primarily via the faeces.
The highest residue levels are found in fat.
At a dose rate of 0.2mg ivermectin per kilogram of bodyweight, plasma levels of ivermectin reach a mean Cmax concentration of 40.44ng/ml and a mean Tmax at 8.35 hours.
This peak falls off gradually to an average level of 3 ng/ml at 10 days.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Maize oil
Polysorbate 80
Apple flavour
Colloidal anhydrous silica
6.2 Major incompatibilities
None known.
6.3 Shelf-life
Shelf-life of the veterinary medicinal product as packaged for sale: 2 years.
To be used immediately after first opening the oral syringe.
6.4 Special precautions for storage
This veterinary medicinal product does not require any special storage conditions.
6.5 Nature and composition of immediate packaging
High density polyethylene pre-filled dose-graduated disposable syringe containing 6.42 g (smaller syringe) or 8.56 g (bigger syringe) of product.
6.6 Special precautions for the disposal of unused veterinary medicinal products or waste materials derived from the use of such products
EXTREMELY DANGEROUS TO FISH AND AQUATIC LIFE.
Do not contaminate surface waters or ditches with product or used containers.
Any unused product or waste material should be disposed of in accordance with national requirements.
7 MARKETING AUTHORISATION HOLDER
Bimeda Animal Health Limited
2, 3 & 4 Airton Close Airton Road
Tallaght
Dublin 24
Ireland
8 MARKETING AUTHORISATION NUMBER(S)
VPA22033/049/001
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 05 September 2003
Date of last renewal: 21 November 2008
10 DATE OF REVISION OF THE TEXT
October 2021
Ivermectin is a semi-synthetic antiparasitic medication derived from avermectins, a class of highly active broad-spectrum antiparasitic agents isolated from the fermentation products of Streptomyces avermitilis.
Ivermectin itself is a mixture of two avermectins, comprising roughly 90% 5-O-demethyl-22,23-dihydroavermectin A1a (22,23-dihydroavermectin B1a) and 10% 5-O-demethyl-25-de(1-methylpropyl)-22,23-dihydro-25-(1-methylethyl) avermectin A1a (22,23-dihydroavermectin B1b).
Pharmacodynamics
Ivermectin is a semisynthetic, anthelminitic agent. It is an avermectin, a group of pentacyclic sixteen-membered lactones (i.e., a macrocyclic lactone disaccharide) derived from the soil bacterium Streptomyces avermitilis. Avermectins are potent and broad-spectrum anti-parasitic agents.
Mechanism of action
Ivermectin binds selectively and with high affinity to glutamate-gated chloride ion channels in invertebrate muscle and nerve cells of the microfilaria.
This binding causes an increase in the permeability of the cell membrane to chloride ions and results in hyperpolarization of the cell, leading to paralysis and death of the parasite.
Ivermectin also is believed to act as an agonist of the neurotransmitter gamma-aminobutyric acid (GABA), thereby disrupting GABA-mediated central nervous system (CNS) neurosynaptic transmission.
Ivermectin may also impair normal intrauterine development of O. volvulus microfilariae and may inhibit their release from the uteri of gravid female worms.
Here at Agridirect we have joined forces with DPD to ensure all packages are delivered promptly and safely to you. We ship to all mainland countries within the EU. Deliveries take place Monday to Friday excluding bank holidays. Once your order has been dispatched from our warehouse you will be notified by email. If there is a delay with your order for any reason you will be contacted immediately.
Due to Brexit we are temporarily unable to ship to the UK. Shipping to Northern Ireland will remain in place.
| Ireland (ROI & NI) | EU (Mainland Only) |
| 2-4 Working Days | 4-6 Working Days |
Some products have an extended delivery time, this is noted on the products.
| Country | Orders Under €129 | Orders Over €129 |
| Ireland (ROI & NI) | €7.99 | €0.00 |
| Austria | €39.99 | €32.00 |
| Belgium | €36.99 | €29.00 |
| Czech Republic | €39.99 | €32.00 |
| Denmark | €39.99 | €32.00 |
| Finland | €51.99 | €45.00 |
| France | €36.99 | €29.00 |
| Germany | €36.99 | €29.00 |
| Hungary | €43.99 | €37.00 |
| Italy | €49.99 | €42.00 |
| Luxenburg | €36.99 | €29.00 |
| Netherlands | €36.99 | €29.00 |
| Poland | €36.99 | €29.00 |
| Portugal | €51.99 | €45.00 |
| Slovakia | €43.99 | €37.00 |
| Slovenia | €43.99 | €37.00 |
| Spain | €49.99 | €42.00 |
| Sweden | €49.99 | €42.00 |
A selection of the products we sell are only licensed for sale within the Republic Of Ireland and can not be shipped outside of the country. These products are noted as only being available within the Republic of Ireland on the individual product pages.
There may be an addition charge on certain bulky items. This charge will be clearly marked on an applicable products and will be explained on the checkout page before payment has been made.
We’re sorry your purchase didn’t work out. But don’t worry; we have a great returns policy to help you out.
All purchases can be returned to us within 14 days of delivery and returned goods must be received within 14 days from the date you informed us of the return.
Purchases may be opened for inspection but must not be used and must be repackaged securely in the original packaging if you wish to return it.
If we discover goods have been used or there has been a loss in value of the goods due to damage to the goods, while in your care or whilst being returned to us, we will reduce the amount refunded, which may amount to the full cost of the product, to cover loss of value of goods.
All returns should be complete which includes boxes, manuals and accessories that may have been included with the order.
All returns must be packaged appropriately for shipping, we will not accept responsibility for damages or loss which occur during shipping of a return product.
We accept no responsibility for goods damaged or lost while in transit to us.
We have partnered with DPD to make your returns process easy and secure. simply follow the steps below and bring your package to an official DPD pickup point.
1) All returns must be accompanied with a fully filled out returns form which can be downloaded here.
2) To print off your return label click here or visit www.dpd.ie/returns and follow the on-screen instructions. Make sure and use your order number as your reference.
3) Bring your package to a DPD pickup point. To find your nearest drop off point here.
Once the returned product has been received into our warehouse and been fully inspected a refund will be issued.
If you choose not to use the DPD returns service we recommend that you use a method that can be tracked.
For the return of bulk products please contact us at sales@agridirect.ie
First off, if you have received a damaged electrical product from us, do not plug it in. Any electrical products that are plugged in are deemed ‘as used and accepted’ and are not accepted as returns. All damages must be reported to us via phone or email within 24hours of receipt of goods. Please ensure you check your items upon delivery.
How do I begin the returns process?
If you wish to begin the return process, please email us at sales@agridirect.ie and ensure the following information is included in your email. Your name, phone number, Order id, the item you wish to return, reason for return and if the product is damaged we require photos of the product.
Once you have sent us all required information a member of our team will assess your claim and will contact you as soon as possible. Please hold off on returning products until a member of our team has called you to confirm.
Once the returned product has been returned to us and fully inspected a refund will be issued.
Please Note: A typical timeline for a refund to show in your account is up to 10 working days from the date processed, depending on your bank.
Would you like to send this voucher to the recipient via email?
Yes No